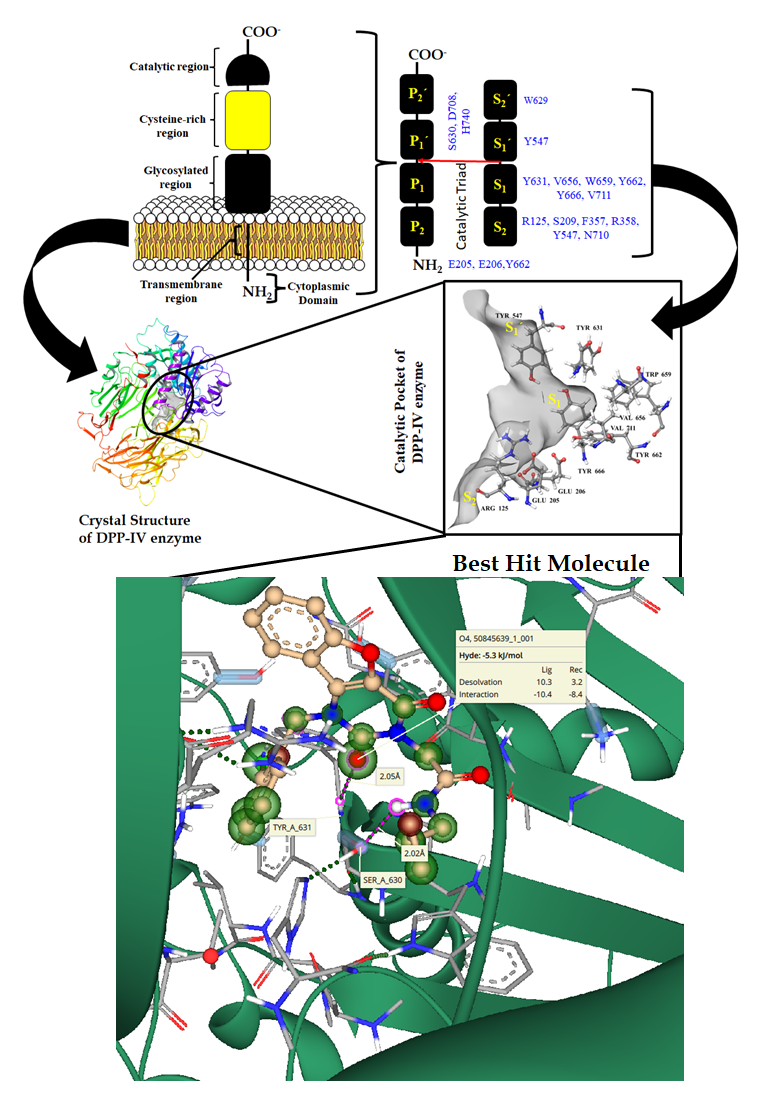
In the present study, I have completed my first milestone by screening several compounds from the ChemLibrary Database, such as PubChem. The molecules were docked into the catalytic pocket of the dipeptidyl peptidase-IV (DPP-IV) enzyme. The best-docked molecules formed a hydrogen bond with the key amino acid residue, i.e., Ser 630 and Tyr 631. Among all the selected compounds from the database, the best-hit molecule has a complementary shape with a catalytic pocket. This molecule (PubChem ID: 50845639) represents the best binding affinity with the amino acids and fits well in the catalytic pocket at the S1 site. All the generated poses of the selected molecule represent the activity in the nM range. A few active molecules represented in nM activities were PubChem_145884560, PubChem_145884561, and PubChem_50845614. In the next objective, molecular dynamic studies will be carried out to understand the protein-ligand stability.
After 3 months, Rakesh Kumar has achieved the following milestones:
- Some active compounds like PubChem_145884560, PubChem_145884561, PubChem ID_50845639, and PubChem_50845614 were identified. Among all these compounds, PubChem ID_50845639 showed remarkable activity and binding with key amino acids in the receptor pocket.
- This milestone will be carried out in the next few months with the best-hit molecules.
- Based on the molecular dynamic stability, the molecule will be purchased, and in vitro studies will be carried out.